Two Secrets[1]
John N. Bahcall
Institute for Advanced Study
Princeton, NJ 08540

I came to Berkeley from Shreveport, Louisiana in June of 1953, 48 years ago, with just enough money to enable me to take a summer course in philosophy. I fell in love with Berkeley, with the intellectual excitement, the physical beauty, and the stimulating people. I wanted to stay for an undergraduate degree. Somehow, my mother persuaded her first cousin to pay for my expenses and tuition and I completed rather quickly the requirements for a degree in philosophy. But, in those days UC Berkeley had an overall requirement that in order to graduate you had to take at least one science course. In high school in Louisiana, I did not take any science course: not chemistry, not physics, not biology. In high school, my job was to play tennis. My faculty advisor at Berkeley said I would have to take a high school science course at night in order to qualify for any of the university science courses. However, I found a relaxed physics professor, Burton Moyer was his name, who agreed to let me take the physics course for physicists that he was teaching even though I had not taken any of the prerequisites. It was the hardest thing I have ever done in my life, but I fell in love with science. I was thrilled by the fact that by knowing some physics you could figure out how real things worked, like sunsets and airplanes, and that after a while everyone agreed on what was the right answer to a question.
As you will hear in a few minutes, my personal story illustrates to some extent the two characteristics of science that I want to emphasize for you tonight.
Your professors have kept secret both these characteristics.
The first secret is that science proceeds as a kind of random walk in the realm of ideas and measurements. It is impossible to predict ahead of time what questions will be most fruitful to try to answer and where the walk will lead. The second secret is that scientists are real people who like to play, who like to have fun. Our work is our hobby. You, as a young scientist, are most likely to make a significant contribution if you work on something that you have fun doing.
These secrets are subversive. They undermine the image of science as a serious activity in which self-sacrificing, ascetic, mature, usually bearded individuals logically go from one step to another in a steady progression toward greater and greater mastery of the physical world. You can therefore understand why your professors have concealed from you the way science is really done. They are worried that if the truth got out, people would be reluctant to pay us for playing around, for having fun.
Anyway, I will be very happy if you remember one sentence from this talk. The sentence I want you to remember is: Science is unpredictable and fun.
I will illustrate this sentence by describing how a debate in the middle of the 19th century between Charles Darwin and Lord Kelvin led, in a very round about way, to evidence for new particle physics at the end of the 20th century.
The debate between Darwin and Kelvin
Our story begins in the middle of the 19th century with a debate between Darwin, the discoverer of the theory of evolution, and Kelvin, the co-discoverer of the second law of thermodynamics. Darwin and the evolutionary geologists of his day believed that the Earth, and therefore the Sun, must be very old. Darwin estimated that the Earth must be more than 300 million years old in order for the observed geological and biological evolutionary processes to have occurred. Lord Kelvin, on the other hand, argued that the Sun, and hence energetic processes on Earth, could not be older than 30 million years. Kelvin’s upper limit for the age of the Sun was at least ten times less than Darwin and evolutionary geologists required. Kelvin obtained a low age for the Sun by arguing that the most efficient known source of energy, gravitation, could supply the luminosity of the Sun for at most 30 million years.
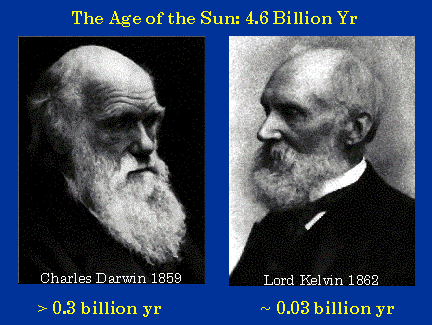
Darwin was shaken and disturbed by the power of Kelvin’s logic and the force of his authority as a brilliant theoretical physicist. In his private correspondence, Darwin admitted that he had no answer to Kelvin’s logic. Darwin removed all mention of time scales in all the subsequent editions of the Origin of the Species after the first edition. We now know that Darwin and the evolutionary biologists and geologists were right; the Sun and Earth are about 4.6 billion years old. Kelvin, despite his brilliant analysis was wrong. We shall now see why.
Albert Einstein contributed the
first key theoretical insight that led to the understanding of how the Sun
shines. While exploring the consequences of the special theory of relativity,
Einstein inferred in 1905 that a little amount of mass could in principle be
converted to a very large amount of energy, i.e., E = mc2.
In 1920, F. W. Aston built a precise mass spectrometer, with which he ultimately measured the masses of many different atoms. He set out to determine the isotopic composition of atmospheric Neon, but he also measured incidentally the masses of hydrogen and helium atoms. His measurements revealed that four hydrogen nuclei, four protons, are heavier than a helium nucleus.
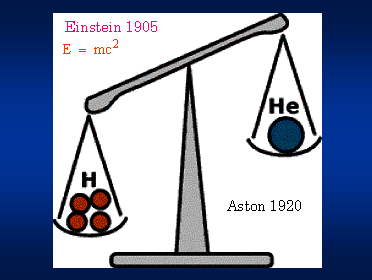
These two discoveries, the theoretical relation between mass and energy and the experimental fact that four hydrogen nuclei weigh more than one helium nucleus, might seem unrelated to the debate between Kelvin and Darwin. Certainly, Einstein and Aston were not thinking about stars when they made their fundamental discoveries. But, Arthur Eddington, the great British astrophysicist,` almost immediately pointed out that the conversion of hydrogen to helium in stars could in principle provide enough energy to keep stars burning for as long as Darwin and the geophysicists required. It took another two decades before the details were worked out.
In 1938, stimulated by a conference at which astronomers and physicists discussed their ideas about how stars shine, Hans Bethe analyzed all the nuclear reactions among light elements that he could think of. He found two sets of reactions which could account for long stellar lifetimes. The basic nuclear reactions involved burning four hydrogen nuclei, four protons, to form a helium nucleus, two positive electrons, and two neutrinos. Hans did not bother to include the neutrinos in the equations that he wrote for the nuclear fusion reactions. He must have thought the neutrinos were so elusive as to not be worth mentioning explicitly. Incidentally, even today, at age 94, Hans Bethe is still enjoying doing theoretical astrophysics.
After the Second World War, Willy Fowler—an experimental nuclear physicist skilled in the techniques of standard nuclear physics measurements—decided that it was more fun trying to measure the laboratory rates for stellar fusion reactions than to make standard nuclear physics measurements. Willy led an international effort to measure the rates of nuclear reactions that Hans Bethe had shown could be important in stars.
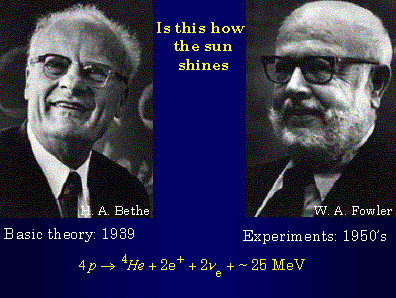
In 1961, now 40 years ago, before most of you were born, Ray Davis (a physical chemist) and I started trying to figure out if there was a direct way to test the theory of how stars shine, to settle experimentally the debate between Darwin and Kelvin. We wanted to find a way to observe the neutrinos that are supposed to be produced deep inside the Sun as hydrogen is burned to helium. We knew that this was a pretty crazy thing for either of us to think about because one can calculate easily that neutrinos can escape from the center of the Sun. This elusiveness would, in principle, allow us to use neutrinos to look inside the Sun and see how solar energy is produced in analogy to what a doctor does when he uses x-rays or ultra-sound to see what is happening inside our bodies.
But, of course, anything that could escape from the center of the Sun would be extremely hard to detect with a reasonable sized experiment on Earth.

Astronomers and physicists generally agreed that solar neutrino experiments were not interesting to think about. Astronomers believed that they understood solar physics and wanted instead to concentrate on frontier problems like how stars explode and produce supernova. The view of physicists was most succinctly summarized by the then Director of Brookhaven National Laboratory, who was fond of saying that: “No astrophysicist can calculate anything with sufficient precision to be of interest to any particle physicist.”
If ever a project seemed unlikely to lead to academic advancement, studying solar neutrinos was it. But, we kept chewing up the possibilities because it seemed like such a neat idea, far out yet a fun thing to think about.
In 1968, as some of you may know, Ray Davis did the experiment with a detector full of cleaning fluid. The result was surprising: Ray’s tank of cleaning fluid captured fewer neutrinos than I had predicted. This became known as the ‘solar neutrino problem.’
I want to diverge from our main story for just a moment to tell you about something that meant a lot to me personally and which is related to the main theme of this talk. When Ray first presented his experimental results at a small, private seminar at Caltech, I was very depressed. I was a young assistant professor, not much older than most of the graduate students here today. I didn’t have tenure and my most widely discussed prediction had just been shown to be wrong. Dick Feynmann saw how discouraged I was and took me for a long walk. To encourage me, he said that I should not feel badly because no one had shown that there was an error in my calculations. This walk, at a very critical time in my life, is an example of the importance of the human element in science. Please, never forget the great importance of the human element in doing science.
By now, many solar neutrino experiments have been performed. They reveal a consistent picture. Neutrinos are observed with the energies and with about the same fluxes as predicted by models of the Sun. The debate between Darwin and Kelvin has been settled experimentally. The Sun shines for a long time using, as Bethe calculated, the energy created by burning hydrogen to helium while producing neutrinos that are measured on Earth.
Sure, there are two or three times fewer solar neutrino interactions than I have calculated on the basis of standard particle and solar physics. But, if anyone had told Ray Davis or me in the 1960’s that there would be 7 experiments that would measure a solar neutrino event rate within a factor of 2 or 3 of the predicted rate: we would have been astonished and delighted!
But, we also found something we weren’t looking for. The discrepancy between standard calculation and experimental detection is robust. Solar neutrinos appear to be telling us about some new particle physics. Something unanticipated happens to the neutrinos after they are created in the center of the Sun.
We have learned how the Sun shines by combining a result of special relativity, E = mc2, with precise measurements of atomic masses, a detailed understanding of nuclear physics, precisely computed models of the Sun, and beautiful experiments that require exquisite engineering, chemistry, and physics.
These unexpected developments represent a random walk in the space of measurements and ideas. Those of us who have been privileged to participate in some of these activities have had a lot of fun working on unconventional ideas and making unusual measurements.
Everything that we have discussed in this talk can be summarized in one sentence. I hope each of you, each of today’s graduates, will have personal experiences that will make this sentence have special meaning for you in your lives. The sentence is: Science is unpredictable and fun.
Have fun! Thank you.
